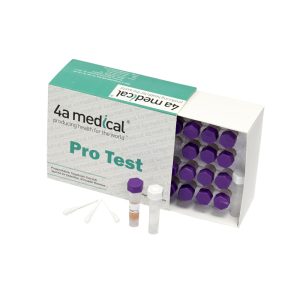
EO BIOLOGICAL INDICATOR
PRODUCT CODE: 4AEOBI
RELATED STANDARDS: 4A Medical Ethylene Oxide Biological Indicator is under the scope of ISO 11138 standard.
INTENDED USE: 4A Medical Ethylene Oxide Biological Indicator is specifically designed for the monitoring of ethylene oxide (EO) sterilization processes. If the sterilization process was not successful, the indicator media will change to yellow after incubation at 37+/-2°C, thus indicating the presence of live Bacillus atrophaeus spores. Incubate the processed biological indicator and the indicator used as positive control for a maximum of 48 hours at 37+/-2°C. Regarding should be made at convenient intervals of 10 hours. A color change to yellow of the growth indicator media means a sterilization process failure has occurred. If after 48 hours there is no color change in the processed indicators, a final negative result is made (the sterilization process was acceptable). The positive control indicator should show a yellow color change for the results to be valid.
OPERATION CONDITIONS: If the sterilization process was successful, the indicator media will remain the original color after incubation. The final readout should be made after 48 hours of incubation at 37+/-2°C.
COMPOSITION: Each tube contains a population of spores of Bacillus atrophaeus ATCC 9372 soaked on a carrier. It also has a growth indicator media contained in the glass ampule.
PACKAGING: 100 pcs in box.







Reviews
There are no reviews yet.