TECHNICAL DATA SHEET
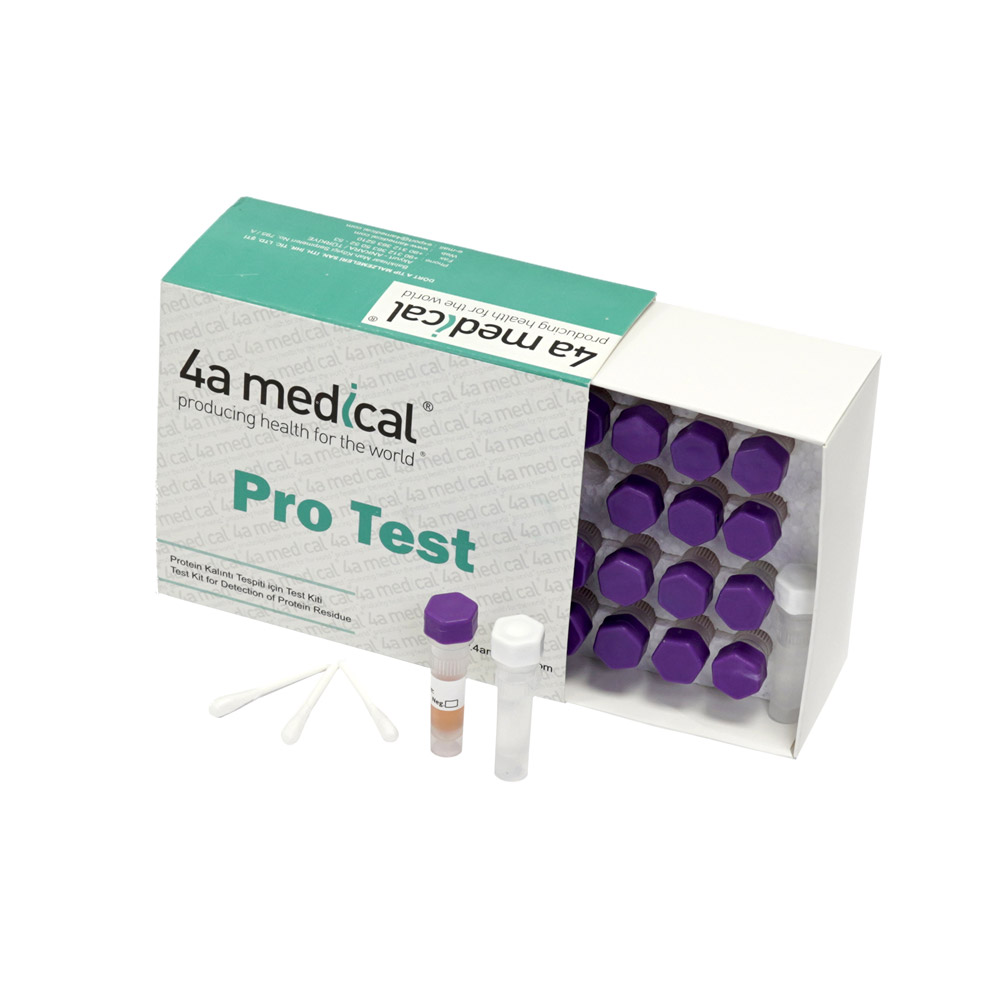
PROTEIN TEST
PRODUCT CODE: 4APRO
RELATED STANDARDS: 4A Medical PRO Test is under the scope of ISO 13485 and 9001 standards.
INTENDED USE: Pro test is used to control whether the cleaning process is successful or not and if there is protein residue on the surface.
OPERATION CONDITIONS: It is necessary to wear gloves in case of contaminations due to the user before starting the analysis. The cotton bud which is present inside the test kit is rubbed onto the parts of the medical and surgical instruments that are suspected to have protein residue, in order to transfer the residue onto the cotton bud. If the residue is dry, it can be soaked with pure water and the process can be repeated. After, the cotton bud is put into the violet-capped tube and shaken for 5 seconds. After shaking, the tube is placed upside down on a flat floor. After 3 minutes, if there is a violet-pink color on the bud or in the fluid, it shows that there is protein on the suspected medical/surgical instrument. An excessive amount of protein on the region that the test is applied may turn the fluid completely violet. The color change after 3 minutes must not be taken into consideration.
STORAGE AND DISPOSAL: Test kit must be stored in a dark room without humidity at 4-20 °C. Expiration date is due to 18 months after the production date. Each box contains 24 pieces.
PACKAGING: 24 pcs in box.
| Product Name | QTY | Size |
|---|---|---|
| Protein Test | 1X | 24 PCS |






Reviews
There are no reviews yet.